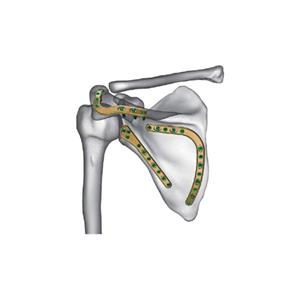
Regulatory frameworks are also evolving
The medical consumables industry is currently experiencing significant developments, driven by technological advancements, regulatory changes, and evolving healthcare needs. Key trends include the increased demand for disposable medical supplies, such as syringes, gloves, and masks, propelled by the ongoing emphasis on infection control and patient safety. The COVID-19 pandemic has spotlighted the importance of these consumables, leading to heightened production and innovation.
Sustainability is becoming a crucial focus, with manufacturers exploring eco-friendly materials and recycling initiatives to reduce environmental impact. Biodegradable and reusable options are gaining traction as healthcare providers aim to balance safety with ecological responsibility.
Regulatory frameworks are also evolving, with stricter guidelines to ensure the quality and safety of medical consumables. This has prompted companies to enhance their compliance measures and invest in research and development to meet these standards. Furthermore, supply chain disruptions experienced during the pandemic have led to a reevaluation of sourcing strategies, with many companies seeking to diversify their suppliers and mitigate risks.
Technological integration is another significant trend, as digital health tools and smart devices are increasingly incorporated into consumables. This includes innovations such as smart syringes that track usage and enhance patient outcomes.
In summary, the medical consumables industry is navigating an era of rapid change marked by increased demand, sustainability efforts, regulatory scrutiny, and technological advancements, positioning itself for a resilient and innovative future in healthcare.